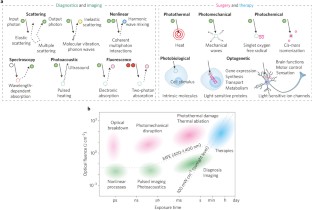
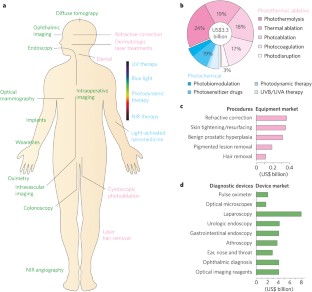
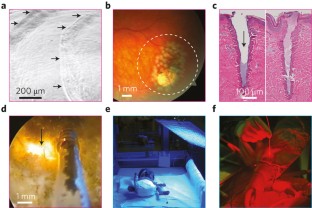
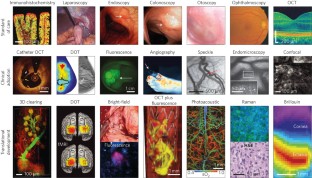
Light in diagnosis, therapy and surgery
Light and optical techniques have made profound impacts on modern medicine, with numerous lasers and optical devices currently being used in clinical practice to assess health and treat disease. Recent advances in biomedical optics have enabled increasingly sophisticated technologies — in particular, those that integrate photonics with nanotechnology, biomaterials and genetic engineering. In this Review, we revisit the fundamentals of light–matter interactions, describe the applications of light in imaging, diagnosis, therapy and surgery, overview their clinical use, and discuss the promise of emerging light-based technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
133,45 € per year
only 11,12 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
References
- Zaret, M. M. et al. Ocular lesions produced by an optical maser (laser). Science134, 1525–1526 (1961). ArticleCASPubMedGoogle Scholar
- Goldman, L. & Wilson, R. G. Treatment of basal cell epithelioma by laser radiation. JAMA189, 773–775 (1964). ArticleCASPubMedGoogle Scholar
- Sakimoto, T., Rosenblatt, M. I. & Azar, D. T. Laser eye surgery for refractive errors. Lancet367, 1432–1447 (2006). ArticlePubMedGoogle Scholar
- Marshall, J., Trokel, S., Rothery, S. & Krueger, R. Long-term healing of the central cornea after photorefractive keratectomy using an exicmer laser. Opthalmology95, 1411–1421 (1988). ArticleCASGoogle Scholar
- Solomon, K. D. et al. LASIK world literature review: quality of life and patient satisfaction. Ophthalmology116, 691–701 (2009). ArticlePubMedGoogle Scholar
- Palanker, D. V. et al. Femtosecond laser-assisted cataract surgery with integrated optical coherence tomography. Sci. Transl. Med.2, 58ra85 (2010). ArticlePubMedGoogle Scholar
- Karabag, R. Y. Retinal tears and rhegmatogenous retinal detachment after intravitreal injections: its prevalence and case reports. Digit. J. Ophthalmol.21, 8–10 (2015). PubMedPubMed CentralGoogle Scholar
- Sternberg, P. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Arch. Ophthalmol.109, 1242–1257 (1991). ArticleGoogle Scholar
- Tanzi, E. L., Lupton, J. R. & Alster, T. S. Lasers in dermatology: four decades of progress. J. Am. Acad. Dermatol.49, 1–34 (2003). ArticlePubMedGoogle Scholar
- Anderson, R. R. & Parrish, J. A. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science220, 524–527 (1983). ArticleCASPubMedGoogle Scholar
- Anderson, R. R. & Parrish, J. A. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg. Med.1, 263–276 (1981). ArticleCASPubMedGoogle Scholar
- Nelson, J. S. et al. Dynamic epidermal cooling during pulsed laser treatment of port-wine stain. A new methodology with preliminary clinical evaluation. Arch. Dermatol.131, 695–700 (1995). ArticleCASPubMedGoogle Scholar
- Fitzpatrick, R. E., Goldman, M. P., Satur, N. M. & Tope, W. D. Pulsed carbon dioxide laser resurfacing of photo-aged facial skin. Arch. Dermatol.132, 395–402 (1996). ArticleCASPubMedGoogle Scholar
- Manstein, D., Herron, G. S., Sink, R. K., Tanner, H. & Anderson, R. R. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg. Med.34, 426–438 (2004). ArticlePubMedGoogle Scholar
- Sherling, M. et al. Consensus recommendations on the use of an erbium-doped 1,550-nm fractionated laser and its applications in dermatologic laser surgery. Dermatologic Surg.36, 461–469 (2010). ArticleCASGoogle Scholar
- Kositratna, G., Evers, M., Sajjadi, A. & Manstein, D. Rapid fibrin plug formation within cutaneous ablative fractional CO2 laser lesions. Lasers Surg. Med.48, 125–132 (2016). ArticlePubMedGoogle Scholar
- Kilmer, S. L. & Anderson, R. R. Clinical use of the Q-switched ruby and the Q-switched Nd:YAG (1064 nm and 532 nm) lasers for treatment of tattoos. J. Dermatol. Surg. Oncol.19, 330–338 (1993). ArticleCASPubMedGoogle Scholar
- Brauer, J. A. et al. Successful and rapid treatment of blue and green tattoo pigment with a novel picosecond laser. Arch. Dermatol.148, 820–823 (2012). ArticlePubMedGoogle Scholar
- Grossman, M. C., Dierickx, C., Farinelli, W., Flotte, T. & Anderson, R. R. Damage to hair follicles by normal-mode ruby laser pulses. J. Am. Acad. Dermatol.35, 889–894 (1996). ArticleCASPubMedGoogle Scholar
- Metelitsa, A. I. & Green, J. B. Home-use laser and light devices for the skin: an update. Semin. Cutan. Med. Surg.30, 144–147 (2011). ArticleCASPubMedGoogle Scholar
- Jackson, S. D. Towards high-power mid-infrared emission from a fibre laser. Nat. Photon.6, 423–431 (2012). ArticleCASGoogle Scholar
- Gilling, P., Cass, C., Cresswell, M. & Fraundorfer, M. Holmium laser resection of the prostate: preliminary results of a new method for the treatment of benign prostatic hyperplasia. Urology47, 48–51 (1996). ArticleCASPubMedGoogle Scholar
- Malek, R. S., Kuntzman, R. S. & Barrett, D. M. High-power potassium-titanyl-phosphate (KTP/532) laser vaporization prostatectomy: 24 hours later. Urology51, 254–256 (1998). ArticleCASPubMedGoogle Scholar
- Sofer, M. et al. Holmium:YAG laser lithotripsy for upper urinary tract calculi in 598 patients. J. Urol.167, 31–34 (2002). ArticlePubMedGoogle Scholar
- Ell, C., Lux, G., Hochberger, J., Müller, D. & Demling, L. Laser lithotripsy of common bile duct stones. Gut29, 746–751 (1988). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wazni, O. et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J. Am. Coll. Cardiol.55, 579–586 (2010). ArticleCASPubMedGoogle Scholar
- Wilkoff, B. L. et al. Pacemaker lead extraction with the laser sheath: results of the pacing lead extraction with the excimer sheath (PLEXES) trial. J. Am. Coll. Cardiol.33, 1671–1676 (1999). ArticleCASPubMedGoogle Scholar
- Grundfest, W. S. et al. Laser ablation of human atherosclerotic plaque without adjacent tissue injury. J. Am. Coll. Cardiol.5, 929–933 (1985). ArticleCASPubMedGoogle Scholar
- Min, R. J., Khilnani, N. & Zimmet, S. E. Endovenous laser treatment of saphenous vein reflux: long-term results. J. Vasc. Interv. Radiol.14, 991–996 (2003). ArticlePubMedGoogle Scholar
- Proebstle, T. M., Moehler, T. & Herdemann, S. Reduced recanalization rates of the great saphenous vein after endovenous laser treatment with increased energy dosing: definition of a threshold for the endovenous fluence equivalent. J. Vasc. Surg.44, 834–839 (2006). ArticlePubMedGoogle Scholar
- Mccoppin, H. H., Hovenic, W. W. & Wheeland, R. G. Laser treatment of superficial leg veins. Dermatologic Surg.37, 729–741 (2011). CASGoogle Scholar
- Hibst, R. & Keller, U. Experimental studies of the application of the Er:YAG laser on dental hard substances: I. Measurement of the ablation rate. Lasers Surg. Med.9, 338–344 (1989). ArticleCASPubMedGoogle Scholar
- Wigdor, H. A. et al. Lasers in dentistry. Lasers Surg. Med.16, 103–133 (1995). ArticleCASPubMedGoogle Scholar
- Strong, M. S. & Jako, G. J. Laser surgery in the larynx. Early clinical experience with continuous CO2 laser. Ann. Otol. Rhinol. Laryngol.81, 792–798 (1972). ArticleGoogle Scholar
- Amin, Z. et al. Hepatic metastases: interstitial laser photocoagulation with real-time US monitoring and dynamic CT evaluation of treatment. Radiology187, 339–347 (1993). ArticleCASPubMedGoogle Scholar
- Mellow, M. H. & Pinkas, H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction: analysis of technical and functional efficacy. Arch. Intern. Med.145, 1443–1446 (1985). ArticleCASPubMedGoogle Scholar
- Wahidi, M. M., Herth, F. J. F. & Ernst, A. State of the art: interventional pulmonology. Chest131, 261–274 (2007). ArticlePubMedGoogle Scholar
- Maisels, M. J. & McDonagh, A. F. Phototherapy for neonatal jaundice. N. Engl. J. Med.358, 920–928 (2008). ArticleCASPubMedGoogle Scholar
- Schwarz, T. & Beissert, S. Milestones in photoimmunology. J. Invest. Dermatol.133, E7–E10 (2013). ArticlePubMedGoogle Scholar
- Gläser, R. et al. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J. Allergy Clin. Immunol.123, 1117–1123 (2009). ArticlePubMedCASGoogle Scholar
- Liu, P. T. et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science311, 1770–1773 (2006). ArticleCASPubMedGoogle Scholar
- Kripke, M. L. Antigenicity of murine skin tumors induced by ultraviolet light. J. Natl Cancer Inst.53, 1333–1336 (1974). ArticleCASPubMedGoogle Scholar
- Stapelberg, M. P. F., Williams, R. B. H., Byrne, S. N. & Halliday, G. M. The alternative complement pathway seems to be a UVA sensor that leads to systemic immunosuppression. J. Invest. Dermatol.129, 2694–2701 (2009). ArticleCASPubMedGoogle Scholar
- Lim, H. W. et al. Phototherapy in dermatology: a call for action. J. Am. Acad. Dermatol.72, 1078–1080 (2015). ArticlePubMedGoogle Scholar
- Johnson-Huang, L. M. et al. Effective narrow-band UVB radiation therapy suppresses the IL-23/IL-17 axis in normalized psoriasis plaques. J. Invest. Dermatol.130, 2654–2663 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Stern, R. S. Psoralen and ultraviolet A light therapy for psoriasis. N. Engl. J. Med.357, 682–690 (2007). ArticleCASPubMedGoogle Scholar
- Norval, M. & Halliday, G. M. The consequences of UV-induced immunosuppression for human health. Photochem. Photobiol.87, 965–977 (2011). ArticleCASPubMedGoogle Scholar
- Becklund, B. R., Severson, K. S., Vang, S.V & DeLuca, H. F. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc. Natl Acad. Sci. USA107, 6418–6423 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Geldenhuys, S. et al. Ultraviolet radiation suppresses obesity and symptoms of metabolic syndrome independently of vitamin D in mice fed a high-fat diet. Diabetes63, 3759–3769 (2014). ArticleCASPubMedGoogle Scholar
- Slusher, T. M. et al. A randomized trial of phototherapy with filtered sunlight in African neonates. N. Engl. J. Med.373, 1115–1124 (2015). ArticleCASPubMedGoogle Scholar
- Anderson, J. L., Glod, C. A., Dai, J., Cao, Y. & Lockley, S. W. Lux vs. wavelength in light treatment of seasonal affective disorder. Acta Psychiatr. Scand.120, 203–212 (2009). ArticleCASPubMedGoogle Scholar
- LeGates, T. A., Fernandez, D. C. & Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci.15, 443–454 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Golden, R. N. et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am. J. Psychiatry162, 656–662 (2005). ArticlePubMedGoogle Scholar
- Lockley, S. W., Brainard, G. C. & Czeisler, C. A. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J. Clin. Endocrinol. Metab.88, 4502–4505 (2003). ArticleCASPubMedGoogle Scholar
- Lam, R. W. et al. Efficacy of bright light treatment, fluoxetine, and the combination in patients with nonseasonal major depressive disorder. JAMA Psychiatry73, 56–63 (2015). ArticleGoogle Scholar
- Dai, T. et al. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob. Agents Chemother.57, 1238–1245 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Dai, T. et al. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond?. Drug Resist. Updates15, 233–236 (2012). ArticleGoogle Scholar
- Wu, P. C., Tsai, C. L., Wu, H. L., Yang, Y. H. & Kuo, H. K. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology120, 1080–1085 (2013). ArticlePubMedGoogle Scholar
- Smith, E. L., Hung, L. F. & Huang, J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Investig. Ophthalmol. Vis. Sci.53, 421–428 (2012). ArticleGoogle Scholar
- Wang, J., Li, B. & Wu, M. X. Effective and lesion-free cutaneous influenza vaccination. Proc. Natl Acad. Sci. USA112, 5005–5010 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Avci, P., Gupta, G. K., Clark, J., Wikonkal, N. & Hamblin, M. R. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg. Med. Surg. Med.46, 144–151 (2014). ArticleGoogle Scholar
- Chung, H. et al. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng.40, 516–533 (2012). ArticlePubMedGoogle Scholar
- Chow, R. T., Johnson, M. I., Lopes-Martins, R. A. & Bjordal, J. M. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet374, 1897–1908 (2009). ArticlePubMedGoogle Scholar
- Naeser, M. A. et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J. Neurotrauma31, 1008–1017 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Arany, P. R. et al. Photoactivation of endogenous latent transforming growth factor-β1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med.6, 238ra69 (2014). ArticlePubMedPubMed CentralCASGoogle Scholar
- Shapiro, M. G., Homma, K., Villarreal, S., Richter, C.-P. & Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nat. Commun.3, 736 (2012).
- Jenkins, M. W. et al. Optical pacing of the embryonic heart. Nat. Photon.4, 623–626 (2010). ArticleCASGoogle Scholar
- Teudt, I. U., Nevel, A. E., Izzo, A. D., Walsh, J. T. & Richter, C.-P. Optical stimulation of the facial nerve: a new monitoring technique?. Laryngoscope117, 1641–1647 (2007). ArticlePubMedPubMed CentralGoogle Scholar
- Wollensak, G., Spoerl, E. & Seiler, T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J. Cataract Refract. Surg.29, 1780–1785 (2003). ArticlePubMedGoogle Scholar
- Lang, N. et al. A blood-resistant surgical glue for minimally invasive repair of vessels and heart defects. Sci. Transl. Med.6, 218ra6 (2014). ArticlePubMedPubMed CentralCASGoogle Scholar
- Roche, E. T. et al. A light-reflecting balloon catheter for atraumatic tissue defect repair. Sci. Transl. Med.7, 306ra149 (2015). ArticlePubMedGoogle Scholar
- Du, Y., Lo, E., Ali, S. & Khademhosseini, A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc. Natl Acad. Sci. USA105, 9522–9527 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hillel, A. T. et al. Photoactivated composite biomaterial for soft tissue restoration in rodents and in humans. Sci. Transl. Med.3, 93ra67 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Castano, A. P., Mroz, P. & Hamblin, M. R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer6, 535–545 (2006). ArticleCASPubMedPubMed CentralGoogle Scholar
- Agostinis, P. et al. Photodynamic therapy of cancer: an update. CA Cancer J.Clin.61, 250–281 (2011). ArticlePubMedPubMed CentralGoogle Scholar
- Spring, B. Q., Rizvi, I., Xu, N. & Hasan, T. The role of photodynamic therapy in overcoming cancer drug resistance. Photochem. Photobiol. Sci.14, 1476–1491 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wan, M. T. & Lin, J. Y. Current evidence and applications of photodynamic therapy in dermatology. Clin. Cosmet. Investig. Dermatol.7, 145–163 (2014). PubMedPubMed CentralGoogle Scholar
- Lozano, M., Cid, J. & Müller, T. H. Plasma treated with methylene blue and light: clinical efficacy and safety profile. Transfus. Med. Rev.27, 235–240 (2013). ArticlePubMedGoogle Scholar
- Yang, Y. et al. Thienopyrrole-expanded BODIPY as a potential NIR photosensitizer for photodynamic therapy. Chem. Commun.49, 3940–3942 (2013). ArticleCASGoogle Scholar
- Idris, N. M. et al. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med.18, 1580–1585 (2012). ArticleCASPubMedGoogle Scholar
- Kim, Y. R. et al. Bioluminescence-activated deep-tissue photodynamic therapy of cancer. Theranostics5, 805–817 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hildebrandt, B. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol.43, 33–56 (2002). ArticlePubMedGoogle Scholar
- Jin, C. S., Lovell, J. F., Chen, J. & Zheng, G. Ablation of hypoxic tumors with dose-equivalent photothermal, but not photodynamic, therapy using a nanostructured porphyrin assembly. ACS Nano7, 2541–2550 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Huang, X., El-Sayed, I. H., Qian, W. & El-Sayed, M. A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc.128, 2115–2120 (2006). ArticleCASPubMedGoogle Scholar
- Cheng, L., Yang, K., Chen, Q. & Liu, Z. Organic stealth nanoparticles for highly effective in vivo near-infrared photothermal therapy of cancer. ACS Nano6, 5605–5613 (2012). ArticleCASPubMedGoogle Scholar
- Lovell, J. F. et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater.10, 324–332 (2011). ArticleCASPubMedGoogle Scholar
- Shaikh, N., Hoberman, A., Kaleida, P. H., Ploof, D. L. & Paradise, J. L. Diagnosing otitis media — otoscopy and cerumen removal. N. Engl. J. Med.362, e62 (2010).
- Thangaratinam, S., Brown, K., Zamora, J., Khan, K. S. & Ewer, A. K. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet379, 2459–2464 (2012). ArticlePubMedGoogle Scholar
- Boas, D. A., Elwell, C. E., Ferrari, M. & Taga, G. Twenty years of functional near-infrared spectroscopy: introduction for the special issue. Neuroimage85, 1–5 (2014). ArticlePubMedGoogle Scholar
- Schwarz, R. A. et al. Noninvasive evaluation of oral lesions using depth-sensitive optical spectroscopy. Cancer115, 1669–1679 (2009). ArticlePubMedGoogle Scholar
- Humeau-Heurtier, A., Guerreschi, E., Abraham, P. & Mahé, G. Relevance of laser doppler and laser speckle techniques for assessing vascular function: state of the art and future trends. IEEE Trans. Biomed. Eng.60, 659–666 (2013). ArticleCASPubMedGoogle Scholar
- Bolay, H. et al. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat. Med.8, 136–142 (2002). ArticleCASPubMedGoogle Scholar
- Boppart, S. A. & Richards-Kortum, R. Point-of-care and point-of-procedure optical imaging technologies for primary care and global health. Sci. Transl. Med.6, 253rv2 (2014).
- Greenbaum, A. et al. Wide-field computational imaging of pathology slides using lens-free on-chip microscopy. Sci. Transl. Med.6, 267ra175 (2014). ArticlePubMedGoogle Scholar
- Shen, L., Hagen, J. A. & Papautsky, I. Point-of-care colorimetric detection with a smartphone. Lab Chip12, 4240–4243 (2012). ArticleCASPubMedGoogle Scholar
- Ming, K. et al. Integrated quantum dot barcode smartphone optical device for wireless multiplexed diagnosis of infected patients. ACS Nano9, 3060–3074 (2015). ArticleCASPubMedGoogle Scholar
- Ambrosio, M. V. D. et al. Point-of-care quantification of blood-borne filarial parasites with a mobile phone microscope. Sci. Transl. Med.7, 286re4 (2015). ArticlePubMedGoogle Scholar
- Bao, J. & Bawendi, M. G. A colloidal quantum dot spectrometer. Nature523, 67–70 (2013). ArticleCASGoogle Scholar
- Shelton, R. L. et al. Optical coherence tomography for advanced screening in the primary care office. J. Biophotonics7, 525–533 (2014). ArticlePubMedGoogle Scholar
- de la Rica, R. & Stevens, M. M. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotech.8, 1759–1764 (2012). Google Scholar
- Chen, Z. et al. Protein microarrays with carbon nanotubes as multicolor Raman labels. Nat. Biotechnol.26, 1285–1292 (2008). ArticleCASPubMedGoogle Scholar
- Armani, A. M., Kulkarni, R. P., Fraser, S. E., Flagan, R. C. & Vahala, K. J. Detection with optical microcavities. Science317, 783–787 (2007). ArticleCASPubMedGoogle Scholar
- Fan, X. & Yun, S.-H. The potential of optofluidic biolasers. Nat. Methods11, 141–147 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Crosetto, N., Bienko, M. & van Oudenaarden, A. Spatially resolved transcriptomics and beyond. Nat. Rev. Genet.16, 57–66 (2015). ArticleCASPubMedGoogle Scholar
- Friedman, A. A., Letai, A., Fisher, D. E. & Flaherty, K. T. Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer15, 747–756 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Veitch, A. M., Uedo, N., Yao, K. & East, J. E. Optimizing early upper gastrointestinal cancer detection at endoscopy. Nat. Rev. Gastroenterol. Hepatol.12, 660–667 (2015). ArticlePubMedGoogle Scholar
- Deepak, P. et al. Incremental diagnostic yield of chromoendoscopy and outcomes in inflammatory bowel disease patients with a history of colorectal dysplasia on white-light endoscopy. Gastrointest. Endosc.83, 1005–1012 (2016). ArticlePubMedGoogle Scholar
- Iddan, G., Meron, G., Glukhovsky, A. & Swain, P. Wireless capsule endoscopy. Nature405, 417 (2000).
- Liao, Z., Gao, R., Xu, C. & Li, Z.-S. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest. Endosc.71, 280–286 (2010). ArticlePubMedGoogle Scholar
- Drexler, W. & Fujimoto, J. G. State-of-the-art retinal optical coherence tomography. Prog. Retin. Eye Res.27, 45–88 (2008). ArticlePubMedGoogle Scholar
- Huang, D. et al. Optical coherence tomography. Science254, 1178–1181 (1991). ArticleCASPubMedPubMed CentralGoogle Scholar
- Yun, S. H. et al. Comprehensive volumetric optical microscopy in vivo. Nat. Med.12, 1429–1433 (2006). ArticleCASPubMedPubMed CentralGoogle Scholar
- Tearney, G. J. et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol.59, 1058–1072 (2012). ArticlePubMedGoogle Scholar
- Prati, F. et al. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur. Heart J.33, 2513–2520 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Bouma, B. E., Tearney, G. J., Compton, C. C. & Nishioka, N. S. High-resolution imaging of the human esophagus and stomach in vivo using optical coherence tomography. Gastrointest. Endosc.51, 467–474 (2000). ArticleCASPubMedGoogle Scholar
- Liu, L. et al. Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Nat. Med.17, 1010–1014 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Yoo, H. et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat. Med.17, 1680–1684 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Roblyer, D. et al. Optical imaging of breast cancer oxyhemoglobin flare correlates with neoadjuvant chemotherapy response one day after starting treatment. Proc. Natl Acad. Sci. USA108, 14626–14631 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Schaafsma, B. E. et al. Optical mammography using diffuse optical spectroscopy for monitoring tumor response to neoadjuvant chemotherapy in women with locally advanced breast cancer. Clin. Cancer Res.21, 577–584 (2015). ArticlePubMedGoogle Scholar
- Jiang, S. et al. Predicting breast tumor response to neoadjuvant chemotherapy with diffuse optical spectroscopic tomography prior to treatment. Clin. Cancer Res.20, 6006–6015 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Fang, Q. et al. Combined optical and X-ray tomosynthesis breast imaging. Radiology258, 89–97 (2011). ArticlePubMedPubMed CentralGoogle Scholar
- Eggebrecht, A. T. et al. Mapping distributed brain function and networks with diffuse optical tomography. Nat. Photon.8, 448–454 (2014). ArticleCASGoogle Scholar
- White, B. R., Liao, S. M., Ferradal, S. L., Inder, T. E. & Culver, J. P. Bedside optical imaging of occipital resting-state functional connectivity in neonates. Neuroimage59, 2529–2538 (2012). ArticlePubMedGoogle Scholar
- Kiesslich, R. et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology127, 706–713 (2004). ArticlePubMedGoogle Scholar
- Buchner, A. M. et al. Comparison of probe-based confocal laser endomicroscopy with virtual chromoendoscopy for classification of colon polyps. Gastroenterology138, 834–842 (2010). ArticlePubMedGoogle Scholar
- Moussata, D. et al. Confocal laser endomicroscopy is a new imaging modality for recognition of intramucosal bacteria in inflammatory bowel disease in vivo. Gut60, 26–33 (2011). ArticlePubMedGoogle Scholar
- Sonn, G. A. et al. Optical biopsy of human bladder neoplasia with in vivo confocal laser endomicroscopy. J. Urol.182, 1299–1305 (2009). ArticlePubMedGoogle Scholar
- Hsiung, P.-L. et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat. Med.14, 454–458 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Sturm, M. B. et al. Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: first-in-human results. Sci. Transl. Med.5, 184ra61 (2013). ArticlePubMedCASGoogle Scholar
- Bird-Lieberman, E. L. et al. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett's esophagus. Nat. Med.18, 315–321 (2012). ArticleCASPubMedGoogle Scholar
- Pan, Y. et al. Endoscopic molecular imaging of human bladder cancer using a CD47 antibody. Sci. Transl. Med.6, 260ra148 (2014). ArticlePubMedCASGoogle Scholar
- Burggraaf, J. et al. Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat. Med.21, 955–961 (2015). ArticleCASPubMedGoogle Scholar
- Fitzgerald, R. Assessing the potential impact of fluorescence angiography in preventing limb loss. Pod. Today 29, http://www.podiatrytoday.com/assessing-potential-impact-fluorescence-angiography-preventing-limb-loss (2016).
- Choi, M., Kwok, S. J. J. & Yun, S. H. In vivo fluorescence microscopy: lessons from observing cell behavior in their native environment. Physiology30, 40–49 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Dimitrow, E. et al. Sensitivity and specificity of multiphoton laser tomography for in vivo and ex vivo diagnosis of malignant melanoma. J. Invest. Dermatol.129, 1752–1758 (2009). ArticleCASPubMedGoogle Scholar
- Palczewska, G. et al. Noninvasive two-photon microscopy imaging of mouse retina and retinal pigment epithelium through the pupil of the eye. Nat. Med.20, 785–789 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Saar, B. G. et al. Video-rate molecular imaging in vivo with stimulated Raman scattering. Science330, 1368–1370 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ji, M. et al. Detection of human brain tumor infiltration with quantitative stimulated Raman scattering microscopy. Sci. Transl. Med.7, 309ra163 (2015).
- Yao, J. et al. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods12, 407–410 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Yang, J.-M. et al. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo. Nat. Med.18, 1297–1302 (2012). ArticleCASPubMedGoogle Scholar
- Galanzha, E. I. et al. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat. Nanotech.4, 855–860 (2009). ArticleCASGoogle Scholar
- Ermilov, S. A. et al. Laser optoacoustic imaging system for detection of breast cancer. J. Biomed. Opt.14, 24007 (2009).
- Kitai, T. et al. Photoacoustic mammography: initial clinical results. Breast Cancer21, 146–153 (2014). ArticlePubMedGoogle Scholar
- Scope, A. et al. In vivo reflectance confocal microscopy of shave biopsy wounds: feasibility of intraoperative mapping of cancer margins. Br. J. Dermatol.163, 1218–1228 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Guitera, P. et al. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases. J. Invest. Dermatol.132, 2386–2394 (2012). ArticleCASPubMedGoogle Scholar
- Scarcelli, G., Besner, S., Pineda, R., Kalout, P. & Yun, S. H. In vivo biomechanical mapping of normal and keratoconus corneas. JAMA Ophthalmol.133, 480–482 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Tsui, C., Klein, R. & Garabrant, M. Minimally invasive surgery: national trends in adoption and future directions for hospital strategy. Surg. Endosc. Other Interv. Tech.27, 2253–2257 (2013). ArticleGoogle Scholar
- Omata, J. et al. Acute gastric volvulus associated with wandering spleen in an adult treated laparoscopically after endoscopic reduction: a case report. Surg. Case Reports2, 47 (2016).
- Jourdan, I. C. et al. Stereoscopic vision provides a significant advantage for precision robotic laparoscopy. Br. J. Surg.91, 879–885 (2004). ArticleCASPubMedGoogle Scholar
- Vahrmeijer, A. L., Hutteman, M., van der Vorst, J. R., van de Velde, C. J. H. & Frangioni, J. V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol.10, 507–518 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Nguyen, Q. T. & Tsien, R. Y. Fluorescence-guided surgery with live molecular navigation—a new cutting edge. Nat. Rev. Cancer13, 653–662 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Widhalm, G. et al. 5-Aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS ONE8, e76988 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Stummer, W. et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol.7, 392–401 (2006). ArticleCASPubMedGoogle Scholar
- Choi, H. S. et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat. Biotechnol.31, 148–153 (2013). ArticleCASPubMedGoogle Scholar
- Hyun, H. et al. Structure-inherent targeting of near-infrared fluorophores for parathyroid and thyroid gland imaging. Nat. Med.21, 192–197 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Verbeek, F. P. R. et al. Near-infrared fluorescence sentinel lymph node mapping in breast cancer: a multicenter experience. Breast Cancer Res. Treat.143, 333–342 (2014). ArticlePubMedGoogle Scholar
- Van Der Vorst, J. R. et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer119, 3411–3418 (2013). ArticleCASPubMedGoogle Scholar
- van Dam, G. M. et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat. Med.17, 1315–1319 (2011). ArticleCASPubMedGoogle Scholar
- Metildi, C. A. et al. Ratiometric activatable cell-penetrating peptides label pancreatic cancer, enabling fluorescence-guided surgery, which reduces metastases and recurrence in orthotopic mouse models. Ann. Surg. Oncol.22, 2082–2087 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Whitney, M. A. et al. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat. Biotechnol.29, 352–356 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Weissleder, R., Tung, C. H., Mahmood, U. & Bogdanov, A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol.17, 375–378 (1999). ArticleCASPubMedGoogle Scholar
- Whitley, M. J. et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci. Transl. Med.8, 320ra4 (2016). ArticlePubMedPubMed CentralCASGoogle Scholar
- Ehlers, J. P. et al. The prospective intraoperative and perioperative ophthalmic imaging with optical coherence tomography (PIONEER) study: 2-year results. Am. J. Ophthalmol.158, 999–1007 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Prati, F. et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l’Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention8, 823–829 (2012). ArticlePubMedGoogle Scholar
- Kut, C. et al. Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci. Transl. Med.7, 292ra100 (2015).
- Ji, M. et al. Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci. Transl. Med.5, 201ra119 (2013). ArticlePubMedPubMed CentralCASGoogle Scholar
- Jermyn, M. et al. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med.7, 274ra19 (2015). ArticleCASPubMedGoogle Scholar
- Celli, J. P. et al. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem. Rev.110, 2795–2838 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Yang, V. X. D., Muller, P. J., Herman, P. & Wilson, B. C. A multispectral fluorescence imaging system: design and initial clinical tests in intra-operative Photofrin-photodynamic therapy of brain tumors. Lasers Surg. Med.32, 224–232 (2003). ArticlePubMedGoogle Scholar
- Ntziachristos, V. et al. Visualization of antitumor treatment by means of fluorescence molecular tomography with an annexin V-Cy5.5 conjugate. Proc. Natl. Acad. Sci. USA101, 12294–12299 (2004). ArticleCASPubMedPubMed CentralGoogle Scholar
- Atreya, R. et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn's disease. Nat. Med.20, 313–318 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Zhang, R. et al. Real-time in vivo Cherenkoscopy imaging during external beam radiation therapy. J. Biomed. Opt.18, 110504 (2013).
- Grotjohann, T. et al. Diffraction-unlimited all-optical imaging and writing with a photochromic GFP. Nature478, 204–208 (2011). ArticleCASPubMedGoogle Scholar
- Chen, B.-C. et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science346, 1257998 (2014).
- Yang, B. et al. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell158, 945–958 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Mosk, A. P., Lagendijk, A., Lerosey, G. & Fink, M. Controlling waves in space and time for imaging and focusing in complex media. Nat. Photon.6, 283–292 (2012). ArticleCASGoogle Scholar
- Doane, T. L. & Burda, C. The unique role of nanoparticles in nanomedicine: imaging, drug delivery and therapy. Chem. Soc. Rev.41, 2885–2911 (2012). ArticleCASPubMedGoogle Scholar
- Youan, B. B. C. Chronopharmaceutical drug delivery systems: hurdles, hype or hope?. Adv. Drug Deliv. Rev.62, 898–903 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Jayakumar, M. K. G., Idris, N. M. & Zhang, Y. Remote activation of biomolecules in deep tissues using near-infrared-to-UV upconversion nanotransducers. Proc. Natl Acad. Sci. USA109, 8483–8488 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Yavuz, M. S. et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mater.8, 935–939 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Carter, K. A. et al. Porphyrin-phospholipid liposomes permeabilized by near-infrared light. Nat. Commun.5, 3546 (2014).
- Li, Y. et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat. Commun.5, 4712 (2014).
- Phillips, E. et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med.6, 260ra149 (2014). ArticlePubMedPubMed CentralCASGoogle Scholar
- Kircher, M. F. et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med.18, 829–834 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Lin, J. et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano7, 5320–5329 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Liu, J. et al. Bismuth sulfide nanorods as a precision nanomedicine for in vivo multimodal imaging-guided photothermal therapy of tumor. ACS Nano9, 696–707 (2015). ArticleCASPubMedGoogle Scholar
- Spring, B. Q. et al. A photoactivable multi-inhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways. Nat. Nanotech.11, 378–387 (2016). ArticleCASGoogle Scholar
- Pasparakis, G., Manouras, T., Vamvakaki, M. & Argitis, P. Harnessing photochemical internalization with dual degradable nanoparticles for combinatorial photo-chemotherapy. Nat. Commun.5, 3623 (2014).
- Lukianova-Hleb, E. Y. et al. On-demand intracellular amplification of chemoradiation with cancer-specific plasmonic nanobubbles. Nat. Med.20, 778–784 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater.1, 16014 (2016).
- Tong, R. & Langer, R. Nanomedicines targeting the tumor microenvironment. Cancer J.21, 314–321 (2015). ArticleCASPubMedGoogle Scholar
- Douglas, S. M., Bachelet, I. & Church, G. M. A logic-gated nanorobot for targeted transport of molecular payloads. Science335, 831–834 (2012). ArticleCASPubMedGoogle Scholar
- von Maltzahn, G. et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat. Mater.10, 545–552 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Dai, B. et al. Programmable artificial phototactic microswimmer. Nat. Nanotech. http://dx.doi.org/10.1038/nnano.2016.187 (2016).
- Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci.8, 1263–1268 (2005). ArticleCASPubMedGoogle Scholar
- Gradinaru, V., Mogri, M., Thompson, K. R., Henderson, J. M. & Deisseroth, K. Optical deconstruction of parkinsonian neural circuitry. Science324, 354–359 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Creed, M., Pascoli, V. J. & Luscher, C. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science347, 659–664 (2015). ArticleCASPubMedGoogle Scholar
- Williams, J. C. & Denison, T. From optogenetic technologies to neuromodulation therapies. Sci. Transl. Med.5, 177ps6 (2013).
- Chow, B. Y. & Boyden, E. S. Optogenetics and translational medicine. Sci. Transl. Med.5, 177ps5 (2013).
- Ramirez, S. et al. Activating positive memory engrams suppresses depression-like behaviour. Nature522, 335–339 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Iyer, S. M. et al. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat. Biotechnol.32, 274–278 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Bruegmann, T. et al. Optogenetic control of contractile function in skeletal muscle. Nat. Commun.6, 7153 (2015).
- Busskamp, V. & Roska, B. Optogenetic approaches to restoring visual function in retinitis pigmentosa. Curr. Opin. Neurobiol.21, 942–946 (2011). ArticleCASPubMedGoogle Scholar
- Barrett, J. M., Berlinguer-Palmini, R. & Degenaar, P. Optogenetic approaches to retinal prosthesis. Vis. Neurosci.31, 345–354 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Kramer, R. H., Mourot, A. & Adesnik, H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat. Neurosci.16, 816–823 (2013). ArticlePubMedPubMed CentralGoogle Scholar
- Polosukhina, A. et al. Photochemical restoration of visual responses in blind mice. Neuron75, 271–282 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Mourot, A. et al. Rapid optical control of nociception with an ion-channel photoswitch. Nat. Methods9, 396–402 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Levitz, J. et al. Optical control of metabotropic glutamate receptors. Nat. Neurosci.16, 507–516 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Gaub, B. M. et al. Restoration of visual function by expression of a light-gated mammalian ion channel in retinal ganglion cells or ON-bipolar cells. Proc. Natl. Acad. Sci. USA111, E5574–E5583 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Melyan, Z., Tarttelin, E. E., Bellingham, J., Lucas, R. J. & Hankins, M. W. Addition of human melanopsin renders mammalian cells photoresponsive. Nature433, 741–745 (2005). ArticleCASPubMedGoogle Scholar
- Ye, H., Daoud-El Baba, M., Peng, R.-W. & Fussenegger, M. A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science332, 1565–1568 (2011). ArticleCASPubMedGoogle Scholar
- Choi, M. et al. Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo. Nat. Photon.7, 987–994 (2013). ArticleCASGoogle Scholar
- Gao, L. et al. Epidermal photonic devices for quantitative imaging of temperature and thermal transport characteristics of the skin. Nat. Commun.5, 4938 (2014).
- White, M. S. et al. Ultrathin, highly flexible and stretchable PLEDs. Nat. Photon.7, 811–816 (2013). ArticleCASGoogle Scholar
- Wang, C. et al. User-interactive electronic skin for instantaneous pressure visualization. Nat. Mater.12, 899–904 (2013). ArticleCASPubMedGoogle Scholar
- Lochner, C. M., Khan, Y., Pierre, A. & Arias, A. C. All-organic optoelectronic sensor for pulse oximetry. Nat. Commun.5, 5745 (2014).
- Koh, A. et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med.8, 366ra165 (2016). ArticlePubMedPubMed CentralCASGoogle Scholar
- Lee, H. et al. An endoscope with integrated transparent bioelectronics and theranostic nanoparticles for colon cancer treatment. Nat. Commun.6, 10059 (2015).
- Mathieson, K. et al. Photovoltaic retinal prosthesis with high pixel density. Nat. Photon.6, 391–397 (2012). ArticleCASGoogle Scholar
- Kim, R.-H. et al. Waterproof AlInGaP optoelectronics on stretchable substrates with applications in biomedicine and robotics. Nat. Mater.9, 929–937 (2010). ArticleCASPubMedGoogle Scholar
- Kim, T. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science340, 211–216 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Montgomery, K. L. et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat. Methods12, 969–974 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Park, S. I. et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol.33, 1280–1286 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Jeong, J.-W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell162, 662–674 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Folcher, M. et al. Mind-controlled transgene expression by a wireless-powered optogenetic designer cell implant. Nat. Commun.5, 5392 (2014).
- Ho, J. S. et al. Wireless power transfer to deep-tissue microimplants. Proc. Natl Acad. Sci. USA111, 7974–7979 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Lee, S. H., Jeong, C. K., Hwang, G.-T. & Lee, K. J. Self-powered flexible inorganic electronic system. Nano Energy14, 111–125 (2014). ArticleCASGoogle Scholar
- Bae, B. et al. Polymeric photosensitizer-embedded self-expanding metal stent for repeatable endoscopic photodynamic therapy of cholangiocarcinoma. Biomaterials35, 8487–8495 (2014). ArticleCASPubMedGoogle Scholar
- Choi, M., Humar, M., Kim, S. & Yun, S.-H. Step-index optical fiber made of biocompatible hydrogels. Adv. Mater.27, 4081–4086 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Nizamoglu, S. et al. Bioabsorbable polymer optical waveguides for deep-tissue photomedicine. Nat. Commun.7, 10374 (2015).
- Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol.33, 277–284 (2015). ArticleCASPubMedGoogle Scholar
- Humar, M. & Yun, S. H. Intracellular microlasers. Nat. Photon.9, 572–576 (2015). ArticleCASGoogle Scholar
- Cho, S., Humar, M., Martino, N. & Yun, S. H. Laser particle stimulated emission microscopy. Phys. Rev. Lett.117, 193902 (2016).
- van Allen, H. W. Some new applications of electricity and light in medicine. N. Engl. J. Med.160, 331–333 (1909). Google Scholar
- Jacques, S. L. Optical properties of biological tissues: a review. Phys. Med. Biol.58, R37–R61 (2013). ArticlePubMedGoogle Scholar
- Moritz, A. R. & Henriques, F. C. J. Studies of thermal injury: II. The relative importance of time and surface temperature in the causation of cutaneous burns. Am. J. Pathol.23, 695–720 (1947). CASPubMedPubMed CentralGoogle Scholar
- Srinivasan, R. Ablation of polymers and biological tissue by ultraviolet lasers. Science234, 559–565 (1986). ArticleCASPubMedGoogle Scholar
- Cain, C. P. et al. ICNIRP guidelines: revision of guidelines on limits of exposure to laser radiation of wavelengths between 400 nm and 1. 4 μm. Health Phys.79, 431–440 (2000). ArticleGoogle Scholar
- Thekaekara, M. P. Solar radiation measurement: techniques and instrumentation. Sol. Energy18, 309–325 (1976). ArticleGoogle Scholar
Acknowledgements
This work was supported by the US National Institutes of Health Director's Pioneer Award (DP1-OD022296) and grants P41-EB015903, R01-EY025454 and R01-CA192878, and National Science Foundation grants ECCS-1505569 and CMMI-1562863.






